Summary
Germ cell tumors (GCT) possess a high activity of telomerase, a ribonucleoprotein complex compensating the erosion of telomeres during cell division by adding TTAGGG-repeats to the telomeric ends of chromosomes. Cisplatin, the most important drug in the treatment of GCT, preferentially acts on G-rich regions like telomeres. Inhibiting telomerase in tumors can result in telomere shortening and senescence and could increase the efficacy of chemotherapy in refractory patients. The study evaluated the promise of the small molecule telomerase inhibitor BIBR1532 as single agent and assessed a possible synergism with cisplatin in a preclinical model of GCT.
GCT-derived cell line 2102EP was cultured with or without 10 μM of BIBR1532. Cell expansion was quantified in population doublings (PD). Telomere length was analyzed by fluorescence in situ hybridization and flow cytometry (flow-FISH). The sensitivity of the cells towards cisplatin was determined by MTT-assay. Telomerase activity was assessed by TRAP assay.
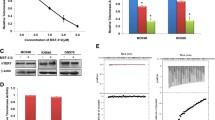
After 300 PD, telomere length diminished from 18.5 kb ± 0.59 kb to 8.9 ± 0.1 kb in BIBR1532 treated 2102 EP cells as compared to 14.5 ± 0.0 kb in untreated control cells. Treated cells did not show altered growth kinetics compared to untreated counterparts. Despite effective shortening of telomeres, the sensitivity of the treated cells towards cisplatin did not increase. Concomitant treatment with BIBR1532 and cisplatin did not result in accelerated telomere shortening.
Telomere length can be shortened significantly by telomerase inhibition in GCT cell line models. However, possibly in view of their extensive telomere “reserve,” telomerase inhibition did neither result in increased sensitivity of 2102 EP cells to cisplatin nor did co-treated cells show accelerated telomere shortening.




Similar content being viewed by others
References
Pottern ML, Brown M, Devesa SS (1998) Epidemiology and pathogenesis of testicular caner. In Ernsthoff MS, Heaney JA, Peschel RE (eds.) Testicular and penile cancer. Blackwell Science, Oxford, pp 2–10.
Bosl GJ, Motzer RJ (1997) Testicular germ-cell cancer. N Engl J Med 337:242–253
Mayer F, Honecker F, Looijenga LH, Bokemeyer C (2003) Towards an understanding of the biological casis of response to cisplatin-based chemotherapy in germ-cell tumors. Ann Oncol 14:825–832
Oosterhuis JW, Looijenga LH (2005) Testicular germ-cell tumours in a broader perspective. Nat Rev Cancer 5:210–222
Blackburn EH (1991) Telomeres. Trends Biochem Sci 16:378–381
Harley CB, Futcher AB, Greider CW (1990) Telomeres shorten during ageing of human fibroblasts. Nature 345:458–460
Greider CW (1998) Telomerase activity, cell proliferation, and cancer. Proc Natl Acad Sci USA 95:90–92
Wright WE, Piatyszek MA, Rainey WE, Byrd W, Shay JW (1996) Telomerase activity in human germline and embryonic tissues and cells. Dev Genet 18:173–179
Albanell J, Bosl GJ, Reuter VE, Engelhardt M, Franco S, Moore MA, Dmitrovsky E (1999) Telomerase activity in germ cell cancers and mature teratomas. J Natl Cancer Inst 91:1321–1326
Skakkebaek NE, Berthelsen JG, Giwercman A, Muller J (1987) Carcinoma-in-situ of the testis: possible origin from gonocytes and precursor of all types of germ cell tumours except spermatocytoma. Int J Androl 10:19–28
Nowak R, Sikora K, Pietas A, Skoneczna I, Chrapusta SJ (2000) Germ cell-like telomeric length homeostasis in nonseminomatous testicular germ cell tumors. Oncogene 19:4075–4078
Kiyozuka Y, Yamamoto D, Yang J, Uemura Y, Senzaki H, Adachi S, Tsubura A (2000) Correlation of chemosensitivity to anticancer drugs and telomere length, telomerase activity and telomerase RNA expression in human ovarian cancer cells. Anticancer Res 20:203–212
Ishibashi T, Lippard SJ (1998) Telomere loss in cells treated with cisplatin. Proc Natl Acad Sci USA 95:4219–4223
Damm K, Hemmann U, Garin-Chesa P, Hauel N, Kauffmann I, Priepke H, Niestroj C, Daiber C, Enenkel B, Guilliard B, Lauritsch I, Muller E, Pascolo E, Sauter G, Pantic M, Martens UM, Wenz C, Lingner J, Kraut N, Rettig WJ, Schnapp A (2001) A highly selective telomerase inhibitor limiting human cancer cell proliferation. EMBO J 20:6958–6968
Wang N, Perkins KL, Bronson DL, Fraley EE (1981) Cytogenetic evidence for premeiotic transformation of human testicular cancers. Cancer Res 41:2135–2140
Sieuwerts AM, Klijn JG, Peters HA, Foekens JA (1995) The MTT tetrazolium salt assay scrutinized: how to use this assay reliably to measure metabolic activity of cell cultures in vitro for the assessment of growth characteristics, IC50-values and cell survival. Eur J Clin Chem Clin Biochem 33:813–823
Koch S, Mayer F, Honecker F, Schittenhelm M, Bokemeyer C (2003) Efficacy of cytotoxic agents used in the treatment of testicular germ cell tumours under normoxic and hypoxic conditions in vitro. Br J Cancer 89:2133–2139
Baerlocher GM, Mak J, Tien T, Lansdorp PM (2002) Telomere length measurement by fluorescence in situ hybridization and flow cytometry: tips and pitfalls. Cytometry 47:89–99
Rufer N, Dragowska W, Thornbury G, Roosnek E, Lansdorp PM (1998) Telomere length dynamics in human lymphocyte subpopulations measured by flow cytometry. Nat Biotechnol 16:743–747
Rufer N, Brummendorf TH, Kolvraa S, Bischoff C, Christensen K, Wadsworth L, Schulzer M, Lansdorp PM (1999) Telomere fluorescence measurements in granulocytes and T lymphocyte subsets point to a high turnover of hematopoietic stem cells and memory T cells in early childhood. J Exp Med 190(2):157–167
Hartmann U, Balabanov S, Ziegler P, Fellenberg J, van der KH, Duyster J, Lipp HP, Bokemeyer C, Kanz L, Brummendorf TH (2005) Telomere length and telomerase activity in the BCR-ABL-transformed murine Pro-B cell line BaF3 is unaffected by treatment with imatinib. Exp Hematol 33:542–549
Gonzalez-Quevedo R, de Juan C, Massa MJ, Sanchez-Pernaute A, Torres A, Balibrea JL, Benito M, Iniesta P (2000) Detection of telomerase activity in human carcinomas using a trap-ELISA method: correlation with hTR and hTERT expression. Int J Oncol 16:623–628
Wu YY, Hruszkewycz AM, Delgado RM, Yang A, Vortmeyer AO, Moon YW, Weil RJ, Zhuang Z, Remaley AT (2000) Limitations on the quantitative determination of telomerase activity by the electrophoretic and ELISA based TRAP assays. Clin Chim Acta 293:199–212
El Daly H, Kull M, Zimmermann S, Pantic M, Waller CF, Martens UM (2005) Selective cytotoxicity and telomere damage in leukemia cells using the telomerase inhibitor BIBR1532. Blood 105:1742–1749
Pascolo E, Wenz C, Lingner J, Hauel N, Priepke H, Kauffmann I, Garin-Chesa P, Rettig WJ, Damm K, Schnapp A (2002) Mechanism of human telomerase inhibition by BIBR1532, a synthetic, non-nucleosidic drug candidate. J Biol Chem 277:15566–15572
Josephson R, Ording CJ, Liu Y, Shin S, Lakshmipathy U, Toumadje A, Love B, Chesnut JD, Andrews PW, Rao MS, Auerbach JM (2007) Qualification of embryonal carcinoma 2102Ep as a reference for human embryonic stem cell research. Stem Cells 25:437–446
Hahn WC, Stewart SA, Brooks MW, York SG, Eaton E, Kurachi A, Beijersbergen RL, Knoll JH, Meyerson M, Weinberg RA (1999) Inhibition of telomerase limits the growth of human cancer cells. Nat Med 5:1164–1170
Ward RJ, Autexier C (2005) Pharmacological telomerase inhibition can sensitize drug-resistant and drug-sensitive cells to chemotherapeutic treatment. Mol Pharmacol 68:779–786
Acknowledgements
We thank Iris Schaefer and Bettina Kirchner for excellent technical assistance. Furthermore we thank our colleagues from Boehringer Ingelheim, namely Klaus Damm for the gift of BIBR1532.
Author information
Authors and Affiliations
Corresponding author
Additional information
Sandra Mueller, Ulrike Hartmann and Frank Mayer contributed equally to the work. Tim H. Brummendorf and Carsten Bokemeyer contributed equally to the work.
Rights and permissions
About this article
Cite this article
Mueller, S., Hartmann, U., Mayer, F. et al. Targeting telomerase activity by BIBR1532 as a therapeutic approach in germ cell tumors. Invest New Drugs 25, 519–524 (2007). https://doi.org/10.1007/s10637-007-9063-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-007-9063-6