Advertisement
Advertisement
October 2016
Lower Extremity Imaging Wish List: What Capabilities Should We Have By 2021?
A look at the future of lower extremity imaging over the next 5 years.
Imaging in peripheral artery disease (PAD) strives to improve both the detection and characterization of disease. The process of detection often begins with a thorough history and physical examination. A patient’s symptoms are usually confirmed and characterized with a pulse examination, but an ankle-brachial index, pulse volume recording, and plethysmography may be required. An arterial duplex ultrasound is then obtained to further characterize the arterial flow with velocities and waveform analysis. Morphologic imaging, such as CT angiography (CTA) or magnetic resonance angiography (MRA), evaluate the vascular tree and not only confirm disease, but better define the extent of disease and allow for a more complete treatment plan. The use of these examinations is reserved for patients undergoing revascularization; however, their additional cost is sometimes controversial. How can preintervention imaging improve over the next 5 years, and how can these imaging modalities become more functional?
IMPROVED CTA IMAGING
CTA imaging is readily available and reproducible; however, there are several limitations that should be improved. In patients with significant vascular disease, the transit of contrast material from the aorta to the distal arteries may be delayed. In addition, transit time varies by patient and between each lower extremity. Along with variable transit time, the amount of enhancement within a vessel is directly related to the amount of iodinated contrast material within the vessel at the time of imaging. Unfortunately, the amount of iodinated contrast material increases the risk of contrast-induced nephropathy, especially in a PAD population that is older and with comorbidities such as hypertension and diabetes.
Improvements to CTA will maximize the contrast-related enhancement in the distal vessels while minimizing the amount of contrast material used, which will allow for more effective peripheral CTA imaging. Ideally, to maximize arterial enhancement, a preliminary small contrast injection is administered to measure the precise flow rate of the contrast from the venous injection site to the popliteal arteries in every patient in order to determine the ideal imaging window. However, this technique is not routinely used because it is technically complicated and utilizes additional contrast material. Instead, most centers use an imaging protocol that is timed relative to the contrast enhancement to the abdominal aorta. The speed of the acquisition of images to the lower extremities is usually predetermined. In patients with PAD and delayed contrast flow into the tibial and pedal vessels, the imaging acquisition may outpace the contrast bolus and generate images with minimal or no vessel enhancement. In these patients, an additional CT acquisition can be quickly performed to obtain this information without the need for additional contrast.
Future technologic changes with regard to CTA should include the ability to perform studies with a variable imaging speed throughout the scan (variable pitch), which would allow for a rapid image acquisition of the aorta and iliac vessels, which typically have very fast flow. The acquisition speed could then be slowed to allow for maximal contrast enhancement of the peripheral vessels. The ability to vary the speed of CT acquisition (pitch) within an examination will allow for the best imaging in large vessels, as well as in the smaller vessels of patients with vascular disease (variable pitch site).
Imaging at Low Kilovolts
The ability to lower the tube voltage (kV) utilized during CT acquisition will allow for improved CTA imaging. Presently, CT scanning is usually performed at 120 to 140 kV, but scanners can deliver a lower tube voltage (80–100 kV), particularly in smaller patients. The lower energy allows for improved enhancement of contrast material within a vessel while reducing the radiation dose exponentially. With this technique, less radiation is administered and contrast enhancement provided by the iodinated contrast is increased without requiring more iodine. This technique will not only increase the quality of the assessment of the contrast-enhanced vessel lumen, but it will also decrease the need for additional iodinated contrast and radiation exposure.1 This technology should become more widespread.
Spectral CT Imaging
The ability of CTA to assess the lumen and vessel wall is an important benefit to aid revascularization. Not only is the nature and extent of the diseased vessel evaluated in larger vessels, CTA provides information on vessel patency and the characteristics of the obstructing plaque. The effect of calcium on subsequent endovascular therapy makes the assessment of vascular calcification as well as the plaque characteristics important. Similarly, if imaging techniques can help identify acute thrombus, they may also further improve patient treatment and outcomes by allowing for better treatment planning.
Moreover, in the small-caliber tibial and pedal vessels, the existence of calcification limits careful assessment of vessel patency. The ability to perform spectral analysis with CT will allow for the assessment of calcium and iodine enhancement separately. The manner in which spectral scanning occurs depends on the particular scanner being utilized. Most systems utilize one of two kinds of energy sources. Systems may use either two x-ray sources (dual source) or a single source that rapidly switches between high and low energy (rapid kW switching). The newest technique uses a single x-ray tube with a unique detector with two layers that detect two different energies simultaneously (detector based).2 Using specific algorithms, the information from both energies is used to create a material-specific image. The advantage of the detector-based technique is that image maps can be created after the scan has been obtained because the detectors are always acquiring the energy information.
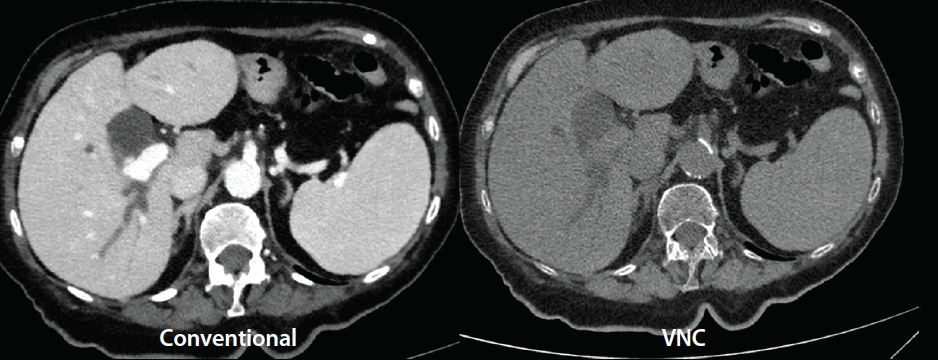
Figure 1. Axial CT images acquired on a detector-based spectral CT system (iQon Spectral CT, Philips Healthcare). The image on the left is the conventional CTA image. The image on the right is a virtual noncontrast (VNC) image obtained by subtracting the iodine-based enhancement from the conventional image.
Courtesy of Philips Healthcare.
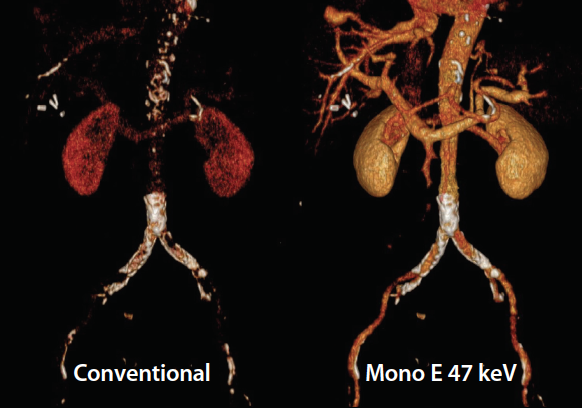
Figure 2. Volume-rendered reconstructions of a patient who underwent a conventional CT scan of the abdomen and pelvis performed on a detector-based spectral CT system (iQon Spectral CT). The image on the left is the conventional CT volume-rendered reconstruction with faint aortic contrast. The image on the right is the contrast-enhanced image where the iodine pixels in the image have been enhanced after its acquisition using the iodine-specific algorithm.
Courtesy of Philips Healthcare.
By using spectral CT imaging, the iodine within a vessel can be isolated to increase the attenuation of iodine. Furthermore, these image maps can be used to accentuate or subtract the iodine in the image, which allows for the ability to increase or decrease vessel attenuation related to iodine. This technique will decrease the need for a noncontrast acquisition by allowing the ability to selectively eliminate the iodine from a CTA and create a virtual noncontrast image (Figure 1). In addition, a conventional CT image with minimal vessel contrast can be converted to a CTA-like image by highlighting the iodine in the vessel (Figure 2). This allows for better assessment of distal vessel patency and quantifying calcified and noncalcified plaque.3 The assessment of vessel patency and the length of the occlusion in a calcified distal tibial vessel has been difficult to assess with conventional CTA.
In our practice, we prefer to perform time-resolved gadolinium-enhanced MRA in patients with calcified small-caliber tibial vessels to best evaluate the tibial vessels. Unfortunately, a limited number of patients can receive MRA examination because of the risk of nephrogenic systemic fibrosis in patients with renal dysfunction. In the future, these patients will hopefully be evaluated with spectral CT to create images without the calcifications, allowing for an assessment of vessel patency.
EXPANDING THE ROLE OF MRA
The use of MRA in patients with severe vascular disease has been limited because of the risk of nephrogenic systemic fibrosis and the dependence on gadolinium chelate contrast agents for this type of imaging. Presently, gadolinium-enhanced MRA sequences are used as first-line sequences to evaluate the peripheral vessels with MRA. These imaging protocols rely on first-pass gadolinium enhancement, rapid image acquisition, and the lack of patient motion. MRA depicts the flow lumen without the issues associated with calcified vessels using CTA. There are novel advancements to improve these studies. Certain sequences allow for the acquisition of a four-dimensional evaluation of a first-pass contrast injection known as time-resolved sequences. There has been significant improvement in imaging protocols to include the use of newer sequences to increase spatial and temporal resolution. Other techniques have helped increase the signal from gadolinium, improving the quality of these MRA sequences. In addition, 3-Tesla MRI scanners have allowed for sequences with more signals within these vessels.
Plaque Evaluation With MRA
The use of MRA to assess the vessel wall has been a challenge. In order to carefully assess a vessel wall, current MRA imaging must be limited to evaluate a particular region, and most research has been performed in the evaluation of carotid plaque.4,5 Over the past decade, longer-lasting gadolinium agents (also called blood pool agents) have allowed for imaging sequences to focus on the wall of the vessels, such as the femoral arteries. Unfortunately, these blood pool agents are not easily obtained. In the future, however, additional techniques to assess plaque should be studied to assess its quality.
Non–Gadolinium-Based MRA
Between now and 2021, non–contrast-enhanced MRA sequences will continue to improve. A number of these sequences currently exist, but their routine use—especially in the lower extremities—is limited. MRA sequences rely on the native signal from flowing blood, but with peripheral vascular disease, the native signal is diminished within the small distal vessels. The greatest change over the next 5 years may be the development on non–gadolinium-based contrast agents for MRA. Current research is evaluating iron oxide–based agents as an alternative to gadolinium agents in patients with renal insufficiency.6,7
Dynamic Image Reconstructions
The use of image processing tools is critical for effective evaluation of imaging studies. These studies typically have a large number of images, and the management, organization, and evaluation of these images is just as important as acquiring them. The ability to evaluate the critical information in an interactive manner for both the interpreting physician and treating physician is important for clinical decision making prior to and during treatment. Further improvement in image processing, manipulation, and access will be required to manipulate large imaging sets.
CAN CTA AND MRA ADD FUNCTIONAL INFORMATION?
In the future, CTA and MRA have the potential to offer much more than simply morphologic information. With flow dynamic modeling, CTA can provide more than just a static snapshot of the vascular tree. In the coronary tree, significant clinical trials over the past 5 years have demonstrated that CTA can be used to assess the hemodynamic significance of a stenosis. Specifically, the measurement of a coronary CTA-generated fractional flow reserve ratio has been investigated to determine the need for subsequent endovascular therapy simply from the derived flow dynamics. In the future, the natural extension of this modeling to the peripheral beds should follow.8
MRA may be used to provide hemodynamic information as well. MRA will not only offer first-pass fluid mechanic modeling, but because of the lack of ionizing radiation, it may also be able to evaluate the perfusion of the associated vascular bed over time to assess the amount of tissue perfusion. The future will hopefully allow for the evaluation of perfusion volume.
IMPROVING IMAGING IN THE ANGIOGRAPHY SUITE
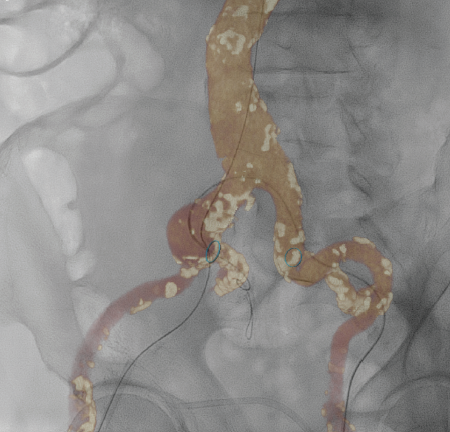
Improvements in lower extremity imaging should continue into the angiographic suite. The ability to use multiple imaging modalities during a procedure will increase. Although new advances have reduced radiation by increasing image quality, the use of ultrasound during revascularization procedures should continue. The ability to evaluate and utilize previously acquired imaging should allow for more accurate treatments. Specifically, an operator should have prior imaging studies available and easily interfaced into the imaging suite. In addition, prior CTA or MRA studies should be available to serve as imaging road maps with the use of fusion technology to reduce contrast and radiation use (Figure 3). In the future, external ultrasound and intravascular ultrasound may be fused into the “workspace volume,” allowing for accurate identification of areas of increased velocity as compared with previous duplex ultrasound.
SUMMARY
The role of lower extremity imaging should continue to increase and help improve the detection and characterization of disease as well as reduce radiation exposure. Real-time image fusion should allow physicians to combine all available imaging data at the time of the procedure. The use of imaging to provide functional information will further increase the value of imaging studies.
1. Ippolito D, Talei Franzesi C, Fior D, et al. Low kV settings CT angiography (CTA) with low dose contrast medium volume protocol in the assessment of thoracic and abdominal aorta disease: a feasibility study. Br J Radiol. 2015;88:20140140.
2. Patino M, Prochowski A, Agrawal M, et al. Material separation using dual-energy CT: current and emerging applications. Radiographics. 2016;36:1087-1105.
3. Korn A, Bender B, Thomas C, et al. Dual energy CTA of the carotid bifurcation: advantage of plaque subtraction for assessment of grade of the stenosis and morphology. Eur J Radiol. 2011;80:e120-125.
4. Demarco JK, Spence JD. Plaque assessment in the management of patients with asymptomatic carotid stenosis. Neuroimaging Clin N Am. 2016;26:111-127.
5. Yuan C, Parker DL. Three-dimensional carotid plaque MR imaging. Neuroimaging Clin N Am. 2016;26:1-12.
6. Hope M, Hope T, Zhu C, et al. Vascular imaging with ferumoxytol as a contrast agent. AJR Am J Roentgenol. 2015:205:W366-373.
7. Walker JP, Nosova E, Sigovan M, et al. Ferumoxytol-enhanced magnetic resonance angiography is a feasible method for the clinical evaluation of lower extremity arterial disease. Ann Vasc Surg. 2015;29:63-68.
8. Ward E, Shiavazzi D, Sood D, et al. CT FFR can identify culprit lesions in aorto-iliac occlusive disease using minimally-invasive techniques [published online ahead of print August 26, 2016]. Ann Vasc Surg.
Constantino S. Peña, MD
Medical Director of Vascular Imaging
Interventional Radiologist
Miami Cardiac and Vascular Institute
Miami, Florida
tinopena@msn.com
Disclosures: None.
Advertisement
Advertisement